Product Description
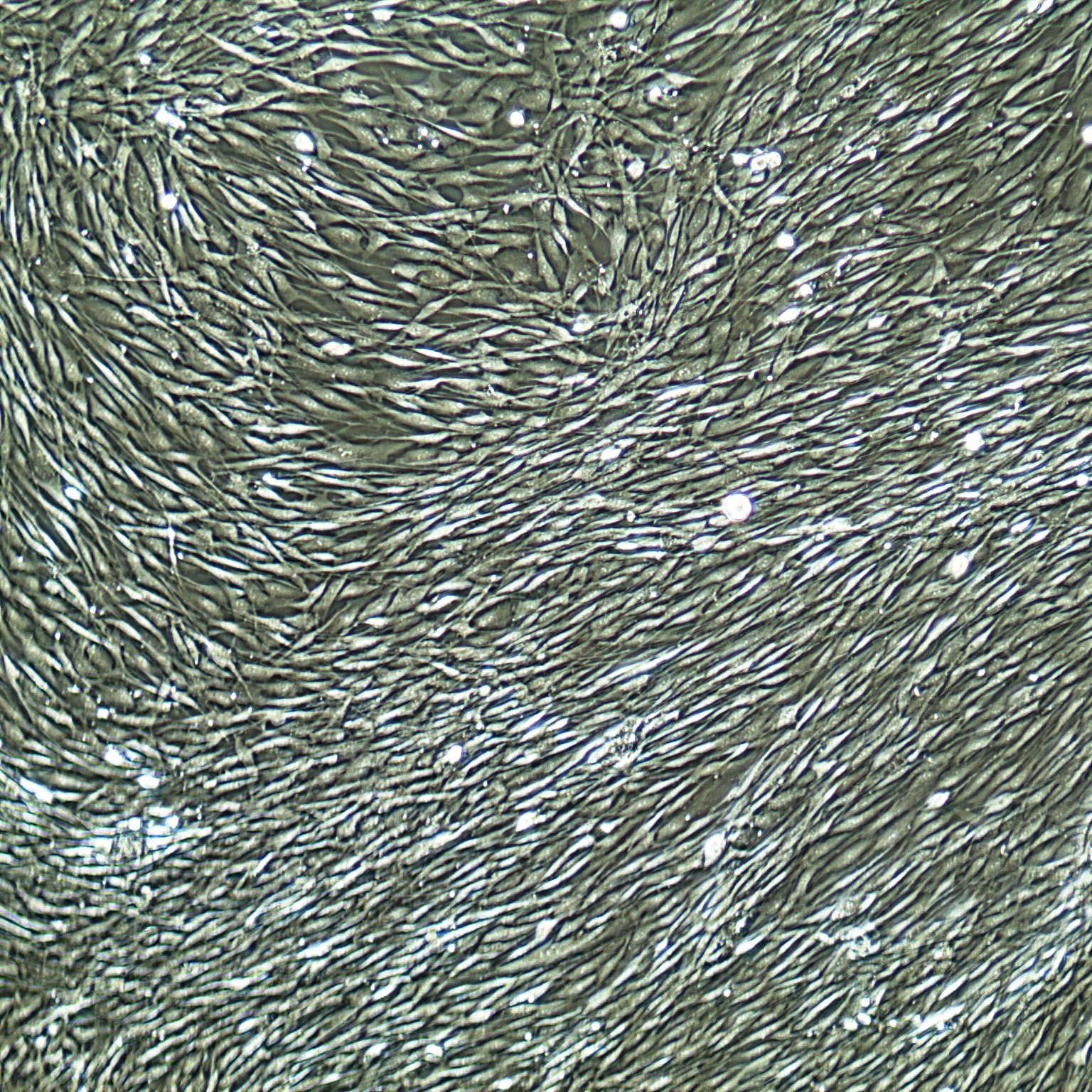
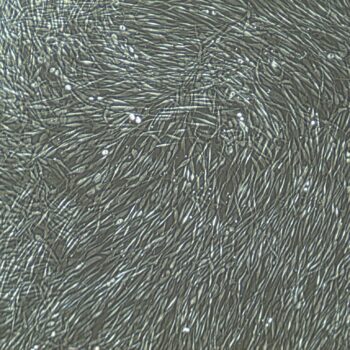
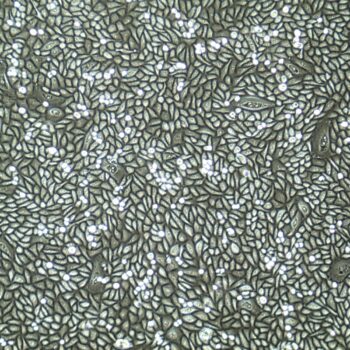
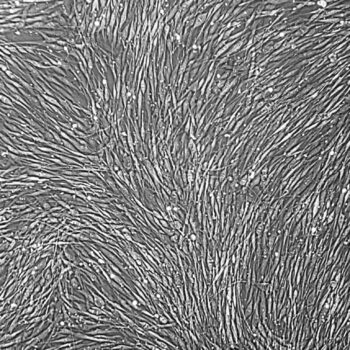
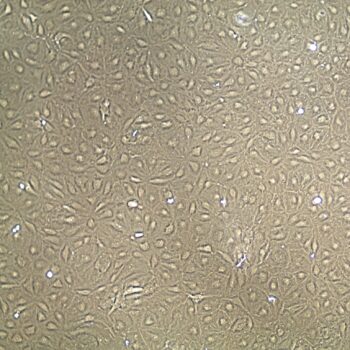
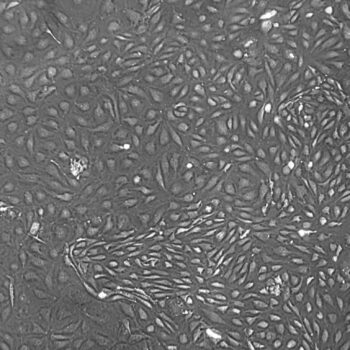
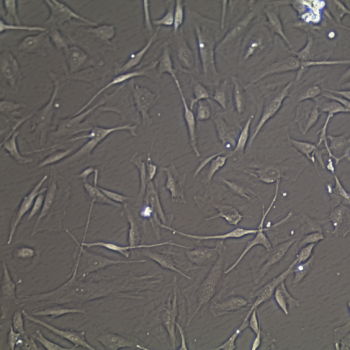
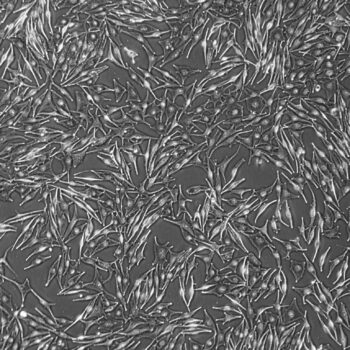
Lifeline® Primary Normal Human Skin Fibroblasts – Neonatal (HDFn) provide an ideal cell system to establish serum free human feeder layers for human embryonic stem cell cultures or as a model to study wound healing, toxicology or basic cell biology.
Primary Dermal Neonatal Fibroblasts are isolated from neonatal human foreskin and cryopreserved after primary culture to ensure the highest viability and plating efficiency.
- Your experimental outcomes will be true, because our Primary Neonatal Skin Fibroblasts are directly cultured from their source organ tissue and have not been modified in any way.
- Your research outcomes will be more representative of the general population, because our Primary Neonatal Dermal Fibroblasts can come from many donors.
- Your experimental results won’t be negatively impacted: Lifeline cells have not been exposed to antimicrobials or phenol red, components which can cause cell stress and “masking effects” that may negatively impact experimental results.
- Our Primary Skin Fibroblasts are quality tested in FibroLife® S2 Low Serum Fibroblast Medium or FibroLife Xeno-Free Frozen Fibroblast Medium to ensure proper growth and morphology over a period of at least 15 population doublings.
- Lifeline Primary Dermal Fibroblasts tested in both FibroLife Serum-Free Medium and FibroLife S2 Medium display optimal growth at rates equal to or greater than classical serum-supplemented medium.
500,000 cells per vial
Primary Dermal Fibroblasts are quality tested for:
- Cell Viability: Minimum 70% viability when thawed from cryopreservation
- Sterility Testing: Negative for mycoplasma. Negative for bacterial and fungal growth.
- Virus Testing: Negative for HIV-1, HIV-2, HBV, and HCV by PCR
Product Specification Sheets
Fibroblast Systems
Skin Cell Systems
SDS and “Safety Data Sheets”
(MSDS or Material Safety Data Sheets)
Cells